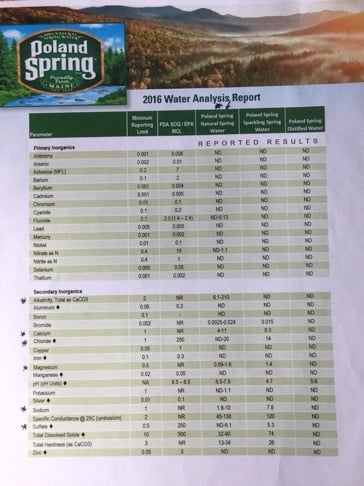
 I am using Poland Springs Spring water for my all grain brewing base. Some of the items listed in the water report are expressed in a “range”. I am trying to become more proficient with Brew’in water so I went on a hunt to find the Bicarbonate level in my water. It is listed as CaCO3, which I need to multiply by 1.22 to get into ppm units. My question is the analysis sheet for the spring water gives me a range of 6.1 to 210, which seems pretty much useless to me. I do test my swimming pool for Alkalinity using the chemical drops etc. Would it be of any use to take a sample of my spring water for each batch and run my pool test on it? Otherwise I guess I could use the distilled water, but I would need to buy 10 individual gallon jugs which is slightly more of a PIA, but as we know we do whatever it takes to make good beer, so is that is what I need to do then so be it.
I am using Poland Springs Spring water for my all grain brewing base. Some of the items listed in the water report are expressed in a “range”. I am trying to become more proficient with Brew’in water so I went on a hunt to find the Bicarbonate level in my water. It is listed as CaCO3, which I need to multiply by 1.22 to get into ppm units. My question is the analysis sheet for the spring water gives me a range of 6.1 to 210, which seems pretty much useless to me. I do test my swimming pool for Alkalinity using the chemical drops etc. Would it be of any use to take a sample of my spring water for each batch and run my pool test on it? Otherwise I guess I could use the distilled water, but I would need to buy 10 individual gallon jugs which is slightly more of a PIA, but as we know we do whatever it takes to make good beer, so is that is what I need to do then so be it.
First what’s wrong with your tap water?
I’ve used 100% distilled and I’ve cut my water 50/50 with distilled when brewing lighter ales and lagers. I honestly see no difference between those and using my tap(well) water and treating it.
If my tap(well) water wasn’t usable I’d use distilled. Mainly for the reason you cite in your OP. Spring water is not as consistent.
One of my local grocery stores has distilled in 2 gal jugs. How is your poland springs packaged?
I used to use my Tap water for extracts and seemed fine. Then moving to all grain I read about all the bad things that can happen… so I researched my tap water a bit and it is a municipal supply drawn from 5 different sources or a mix of them at any given time . So I thought using the spring water would at least give me a reasonable base to build on, and I add salts etc. depending on the profile I want to achieve. Its just the Bicoarbonate that has such a large range. If I can test for that reliably by the batch then I have no problem doing that.
My spring water I can get in 5 gallon bottles or 2.5 gallon, so I typically grab 4 of the 2.5’s and I am on my way. I notice the distilled where I shopped only came in 1 gal jugs.
Maybe for my next batch I will suck it up, get a bigger cart and toss in 10 gal of distilled and see if I notice any difference. A year from now I will prob be filling my cooler with tap water…
I do have a chinook extract batch in the primary that I made with 5 gallons of distilled. I am interested to see if I can detect a difference over tap water on an extract.
If your testing for alkalinity, isn’t that still pH? Trying to understand the components that make water why it is, is difficult. So from a very novice aspect, do use your tap water, treat for chloride with Camden tablets, then to measure pH of your water after you’ve added you salts/acid, but before you add/mash with malt… I’m trying to understand my water and the pH relation before and after malt addition… So far, I can get my water 5.7, 5.8 and after mashing (BIAB style) I can end up at 5.2… I have not done this on a serious attempt with a Pilsner, and I would follow what Danny Boy offers… Too much minerals in my water won’t make for a good pils… Mediocre is where it ends up… For now… Sneezles61
Looking back at your OP…I think you’re confusing alkilinity and bicarb. They’re not the same and I doubt your pool water test kit will test bicarb. Bicarb is HCO3, alkilinity is CaCO3. High bicarb, alkilinity and pH are related but not always directly…I don’t think. Acid reduces all.
I use Brunwater to adjust mash pH. I check the water prior to adjustment, plug that reading into brunwater and make my additions, then a few minutes into the mash I check my pH. Usually shoot for 5.4 mash pH and 5.2 kettle pH for lagers. Lager yeasts don’t tend to lower pH during fermentation as much as ale yeasts.
According to my last Ward Labs water report my well water bicarb is 139 and pH 7.8. Last brew day my meter said the pH was 7.1. Brunwater said my lactic acid addition brought the mash pH to 5.42, bicarb and alkilinity both to negative numbers. This was for a pilsner. In darker beers the acidity of the grain helps reduce the pH.
For Pilsners I find it’s all about adding more lactic acid than you may feel comfortable with but the Pilsen(the city) water profile is very very soft and I’m happy with how mine are turning out.
Read the water knowledge again in regards to bicarb. I learn something every time I read it. Also you could search on here for threads where Martin Brungard has commented on bicarb in brewing water.
In the end do what works for you. Brew a few test batches with all three waters, spring, distilled, treated tap…decide which works best for you then minimize the aggravation and cost of purchased water where ever you can. Just my 2 cents…
I have to figure out with the wide range of bicarbonate in the spring water if that range can present a problem or not, or maybe I just assume it’s in the middle and treat the water based on that. I prob would never notice the difference personally nor would I remember the difference batch to batch unless there was some huge impact one way or the other.
I was confusing the bicarbonate with alkalinity as something I read gave me that idea. Would be nice if I could easily test for it, but I guess that is not the case.
I will most likely try a batch with distilled water and see how it tastes just out of curiosity.
I’m gonna need to invite some people over as I already have 15 gallons in the pipeline in my basement. Gotta buy some additional kegs.
This is the problem I have. Ours is only from two but the numbers are very different. I have an email conversation going with a very helpful guy at our municipal supply so we will see.
I thought about getting it tested but then what happens if they change the supply or the mix? Buying water isn’t practical doing 20 gallons.
Maybe I should put in my own RO system … I guess it would take a while to make 10 gal though. We are thinking of moving to South Carolina some day and I recall from my days at Parris Island that would not be good brewing water. Maybe different today but at the time it was like drinking pool water.
I was reading the water book last night and it talks about total alkalinity being the sum of 3 carbonates. It’s over my head. I would need to get a water for dummies book to start. The bottom line is so far I have not brewed a batch I did not like, so what I am doing now with water is just playing around to learn more.
Thats whats so cool about this hobby, even most mistakes can still put a smile on your face! I’ve made a few mutts… Mix em with another, and you won’t have to dump it!! Sneezles61
Sneezles61
@hd4mark and @tominboston RO is the way to go for what your dealing with. What I did was bought 20gal of distilled water and used it for batches. Then daily I would refill them with my RO water. I’ve now amassed 40 so I can do two 10gal batches and a 5gal batch without worrying too much about water amounts.
Loopie, do you have an RO system installed?
I do. My bro installs and services softeners and RO units so I have a top of the line system that I know is maintained. RO is essentially 98% mineral free so I start with al “zeros” and build my water profile. Your filters and membrane will last longer if it’s installed after a softener.
Edited to add: it’s not mandatory though and with being on city water likely not needed. I’m on city water but as I said.l. My bro installs them so…
^^^^^^ Lucky sh… poop!! Sneezles61
Finally figured out my water. I’ll post a separate thread.
I hope it is wet…  Sneezles61
Sneezles61
Confusing stuff water. Been reading lots of info. About this. Should had spent more time paying attention to chemistry at school. Here on bonaire they. Use ro water. So on my next beer product order gonna buy some chemicals. And start playing with the water