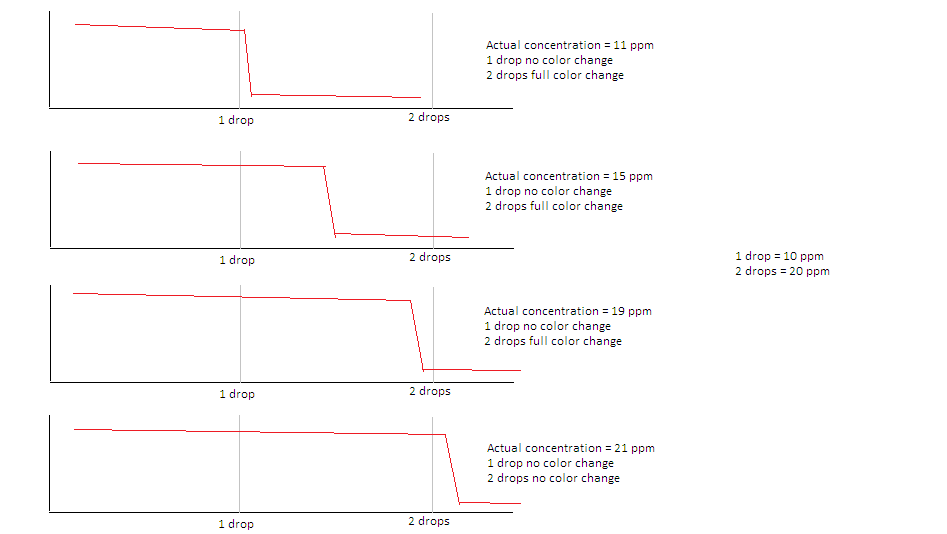
I’ve just starting using the LaMotte BrewLab and am wondering about how to interpret titration test results. In essence if “1 drop =10 ppm” and “2 drops = 20 ppm”, and 1 drop results in no change in color but the 2nd drop results in a full change in color, what is the concentration?
My guess is that it could be anywhere from 11-20 ppm.
I prepared rough graphs to illustrate (I don’t know how closely they would resemble a real titration curve).
For high concentrations a difference of 10 ppm wouldn’t matter much…it is the lower concentrations I am concerned with, since for small concentrations, things like sulfate/chloride ratios can vary widely.